Abstract
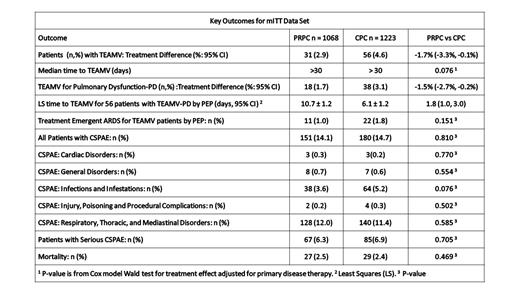
Background. Platelet transfusion is a critical therapy for hematology-oncology patients at risk of transfusion-transmitted infection (TTI) and pulmonary injury. Amotosalen-UVA pathogen reduction (PR) treatment of apheresis platelet components (PC) in plasma or additive solution (INTERCEPT Blood System for Platelets, Cerus, Concord, CA) is FDA approved to reduce risk of TTI and transfusion associated graft vs. host disease (TA-GVHD). PRPC meet the FDA bacteria risk reduction guidance, and approximately 50% of U.S. PC are PRPC. Amotosalen-UVA PR replaces bacteria screening, gamma irradiation, and CMV serology. PR is performed within 24 hours of collection enabling early release of PRPC with 5-day storage. We tested the hypothesis that PRPC were not inferior to conventional PC(CPC) for the incidence of pulmonary injury. Methods. An open-label sequential cohort study in platelet transfusion dependent hematology-oncology patients was conducted under routine practice conditions in 15 clinical centers. Each site enrolled a CPC cohort followed by a PRPC cohort using 4 primary therapy strata matched ± 10%: chemotherapy without hematopoietic cell transplant (HCT), HCT with myeloablation, HCT with non-myeloablative conditioning, and HCT with reduced intensity conditioning (RIC). Patients were supported with the assigned PC type for up to 21 days with 7 days of surveillance after the last PC exposure. Patients participated in only one cohort. The primary endpoint was treatment emergent assisted mechanical ventilation (TEAMV) by intubation or tight mask with positive end expiration pressure (5cm H 2O) after initiation of study PC. All endpoint patients were adjudicated by a blinded pulmonary expert panel (PEP) for diagnosis of acute respiratory distress syndrome (ARDS) by the Berlin Criteria. Secondary endpoints included: time to initiation of TEAMV, clinically significant pulmonary adverse events (CSPAE, CTCAE ≥ Grade 2), transfusion reactions, and mortality. The incidence of TEAMV by non-inferiority (margin = 2.3%), and secondary endpoints were analyzed by modified intention to treat (mITT) and per protocol (PP). Sensitivity analyses with propensity score matching for key variables were conducted for the primary endpoint. The associations between PC and categorical variables were tested by stratified Cochran-Mantel-Haenszel and continuous variables by ANOVA for two-sided significance p = 0.05. results. A total of 2291 pediatric and adult patients (1068 PRPC and 1223 CPC) were enrolled in the respective cohorts with transfusion of 5,277 PRPC and 5,491 CPC. PC assignment compliance and study completion were > 94%. For the mITT data set, the cumulative incidence of TEAMV was lower for the PRPC cohort (log rank p = 0.039) than the CPC cohort (2.9% versus 4.6%, HR = 0.633: 95% CI 0.408-0.982). PRPC by mITT were non-inferior to CPC for the incidence of TEAMV due to all indications, and for TEAMV with pulmonary dysfunction (PD) by PEP (Table). PP analyses were consistent with mITT. Relative risk (RR) of TEAMV showed significantly (p<0.05) decreased RR of PRPC respectively for baseline covariates: age < 65 (0.53), male (0.54), non-white (0.32), chemotherapy (0.40), prior pulmonary disease (0.55), and prior cardiac disease (0.58). Least squares (LS) mean days to initiation of TEAMV for patients with PD were longer for PRPC recipients. PEP adjudicated incidence of ARDS was not significantly different between cohorts (Table). Total and serious CSPAE were not different between the cohorts. There were no significant differences between cohorts in Respiratory, Thoracic, and Mediastinal Disorders, the most frequent system organ class event. Mortality was not different between cohorts. Multivariate analysis (mITT) for the probability of CSPAE or transfusion associated cardiac overload (TACO) showed PC type had no effect. The odds ratio (OR) of CSPAE or TACO during PC support was significantly increased (p< 0.05) in both cohorts for history of cardiac disease (1.35), history of pulmonary disease (2.57), diagnosis of Myelodysplasia (1.88), and diagnosis of Myelodysplasia/Myeloproliferative disease (2.27). There was a significant treatment interaction (p= 0.043) between PC type and acute myelogenous leukemia (AML), increased OR = 1.49 for CPC versus PRPC. Conclusions. PRPC did not potentiate pulmonary injury during PC support; and their use may decrease TEAMV risk with benefit of reduced TTI risk.
Wheeler: Novo Nordisk A/S: Consultancy; Bayer: Consultancy; BioMarin: Consultancy; HEMA Biologics: Consultancy; Spark: Consultancy; Takeda: Consultancy; UniQure: Consultancy. Nooka: Janssen Oncology: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Oncopeptides: Consultancy; Amgen: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Adaptive technologies: Consultancy; GlaxoSmithKline: Consultancy, Other: Travel expenses; Karyopharm Therapeutics: Consultancy. Uhl: UpToDate: Patents & Royalties; Abbott: Consultancy, Speakers Bureau; Grifols: Consultancy, Speakers Bureau. Spinella: Secure Transfusion Services: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Cerus Corporation: Consultancy, Research Funding. Liu: Cerus Corporation: Current Employment, Current equity holder in publicly-traded company. Benjamin: Cerus Corporation: Current Employment, Current equity holder in publicly-traded company. Corash: Cerus Corporation: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal